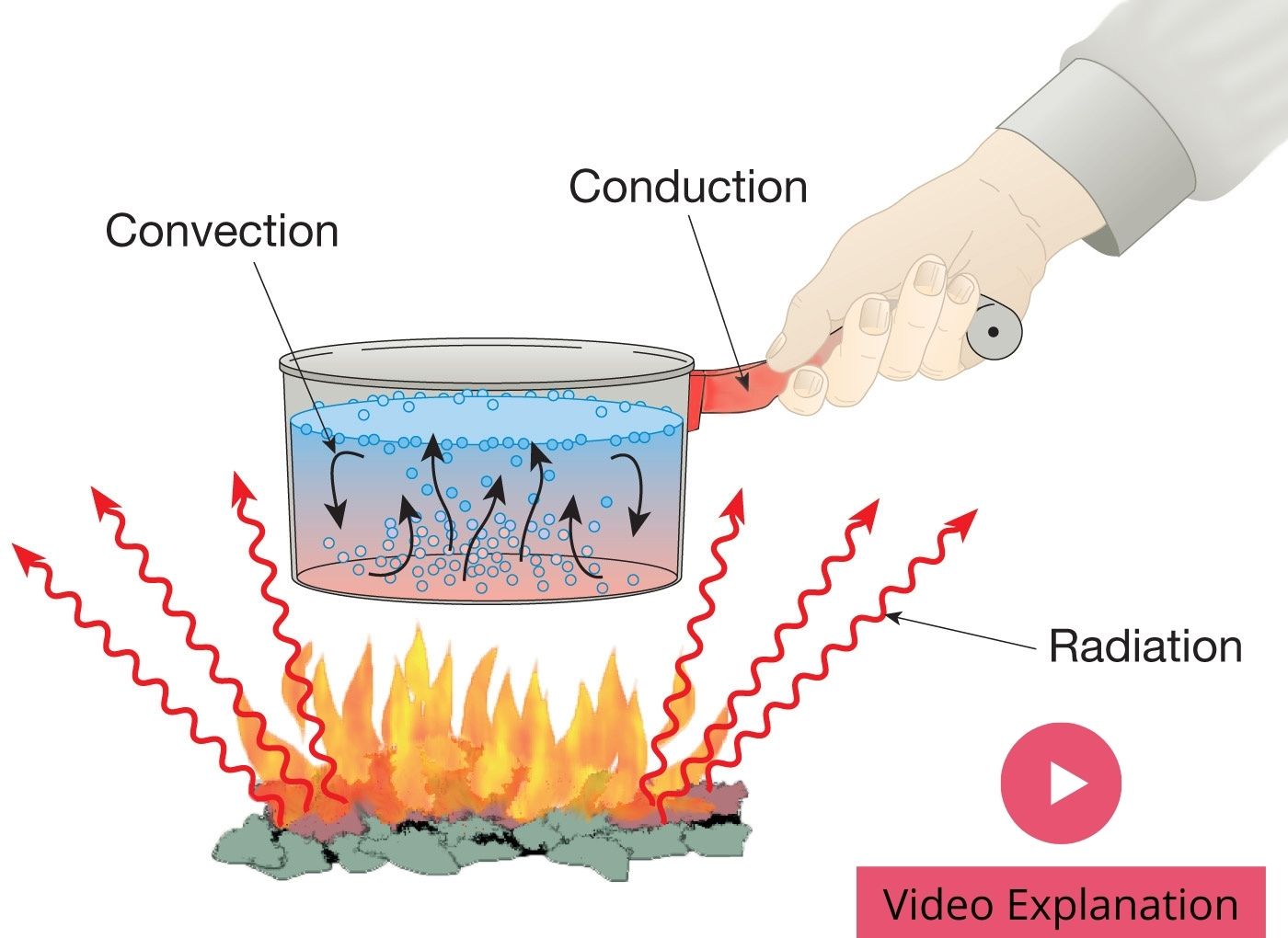
Thermal conduction is the transfer of internal energy by microscopic collisions of particles and movement of electrons within a body. The colliding particles, which include molecules, atoms, and electrons, transfer disorganized microscopic kinetic and potential energy when joined, known as internal energy. Conduction takes place in most phases: solid, liquid, gases and plasma.
Heat spontaneously flows from a hotter to a colder body. For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becoming more uniform.